Date: 14-APRIL-2020 Last Updated: 5-FEBRUARY-2026
Using Acetone as an Organic Modifier in Aqueous Normal Phase (ANP) Chromatography with Cogent TYPE‑C™ Silica Columns
Aqueous Normal Phase (ANP) chromatography on Cogent TYPE‑C™ silica columns is well known for its unique retention mechanism, enabling robust separations of highly polar compounds. One frequently asked question among experienced chromatographers is whether acetone can be used as an alternative to acetonitrile as the organic component of the mobile phase.
The answer is yes—with important qualifications.
This technical note expands significantly on the underlying considerations, detector limitations, retention behavior, and mechanistic implications so that advanced users can make well‑informed decisions during method development.
1. Detector Compatibility: UV vs. LC‑MS/ELS Detection
The most significant constraint on using acetone is UV detection.
Why acetone is unsuitable for UV‑based HPLC methods
Acetone has a low UV cutoff, meaning it absorbs strongly in the low‑wavelength region where most analytes are detected. This introduces baseline interference, reduced sensitivity, and often complete masking of early‑eluting peaks. Therefore:
- Acetone is not compatible with standard UV detection systems.
- It is acceptable only when UV detection is not employed.
This guidance is explicitly stated in the source article.
When acetone is suitable
Acetone performs well with detection technologies not affected by UV absorbance:
- LC‑MS (ESI, APCI, etc.)
- Evaporative Light Scattering (ELS)
- Charged Aerosol Detection (Corona CAD)
MicroSolv confirms that acetone does not present detector‑related issues in these modes.
2. Effects on Retention in ANP Mode
One of the most valuable aspects of switching from acetonitrile to acetone is the differential impact on ANP retention, especially with polar analytes such as amino acids.
Solvent polarity & hydrogen‑bonding differences
Acetone and acetonitrile are both polar, aprotic solvents, but their hydrogen‑bond acceptor strengths and dipole characteristics differ. This affects:
- Interactions with the water layer on TYPE‑C™ silica
- Solute partitioning between the mobile phase and the silica hydride surface
- Resulting electrostatic and adsorption contributions to ANP retention
Empirical retention behavior
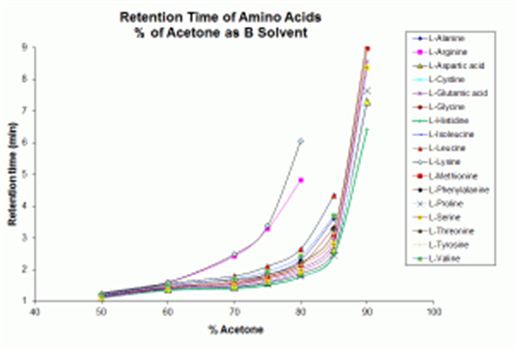
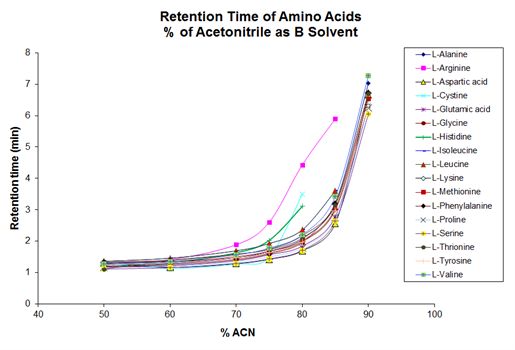
Side‑by‑side retention maps for amino acids (as referenced in the KB article) show:
- Significant shifts in retention for several amino acids, depending on whether acetone or acetonitrile is used.
- Some analytes become better resolved, allowing peak pairs that partially coeluted in one solvent to become baseline‑resolved in the other.
These documented differences make acetone a valuable tool for ANP method development, particularly when dealing with analyte sets exhibiting closely eluting positional or structural isomers.
3. Method Conditions (from the original data)
Flow rate: 0.4 mL / minute
Mobile Phase:
Solvent A: DI Water + 0.1% Formic Acid
Solvent B: Acetonitrile or Acetone + 0.1% Formic Acid
4. Chemical Reactivity Warning: Acetone with Primary Amines
Experienced chromatographers working with primary amines may be aware that acetone can form imines under certain conditions.
Is imine formation a risk in ANP mobile phases?
The reaction requires:
- A primary amine,
- A ketone or aldehyde (acetone),
- An acid catalyst,
- Low water content.
The University of Liverpool’s findings (quoted in the KB article) emphasize that the absence of acid or water dramatically slows or prevents imine formation.
MicroSolv reports that under typical ANP conditions—including 0.1% formic acid in both solvents and high water content—imine formation has not been observed in any of their method development work.
This makes acetone safe for routine use as long as the mobile phase is not anhydrous and includes a mild acidifier (as in the listed method conditions).
5. Practical Recommendations for Technical Users
✔ Use acetone for LC‑MS and aerosol‑based detection
Ideal for workflows prioritizing MS sensitivity, improved desolvation, or matrix handling.
✔ Consider acetone as a method development lever
If a critical pair remains unresolved in acetonitrile-based ANP, switching to acetone may significantly alter selectivity.
✔ Maintain mildly acidic, aqueous conditions
This eliminates concerns about unwanted imine formation when analyzing primary amines.
✔ Document retention shifts carefully
Because acetone and acetonitrile produce distinct selectivity patterns, equilibrating the column thoroughly and documenting changes will ensure reproducibility.

